
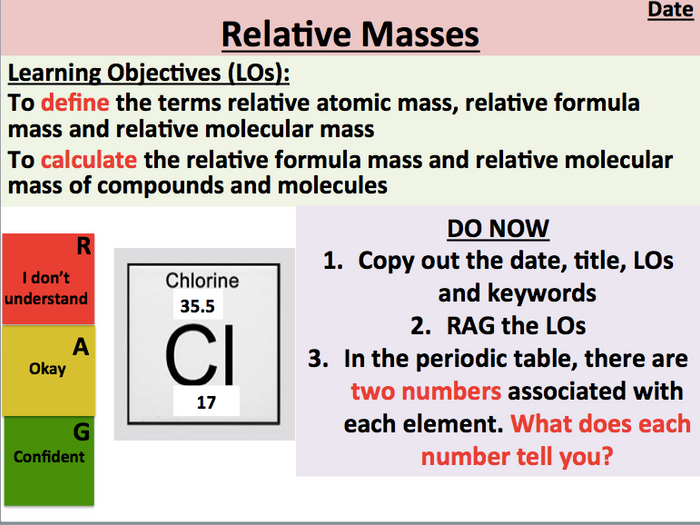
N = number of neutrons How to Find Atomic Mass? This atomic mass equation is similar to the formula of mass number:
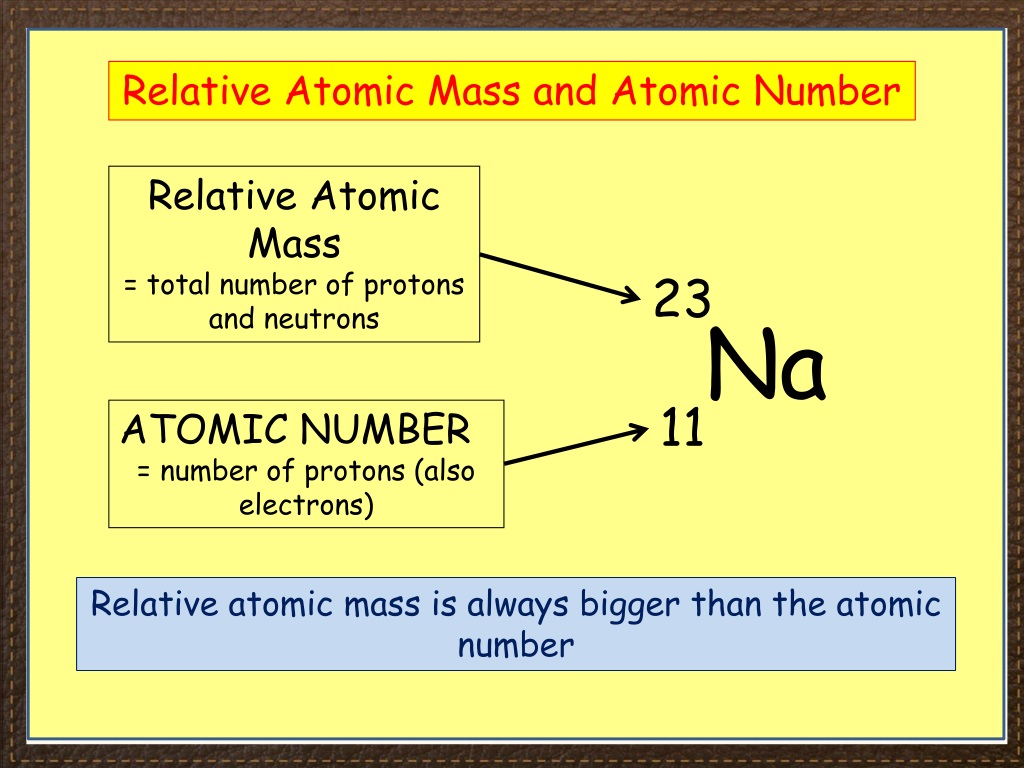
How to calculate atomic mass? According to atomic mass definition, find a reasonable approximation of the atomic mass of an atomic mass unit, we can add the number of protons and neutrons together so that the formula for atomic mass is:Ītomic mass (u) ≈ number of neutrons + number of protons Therefore, the unit of atomic mass is \( 1.66 * 10^-27 kg \). An atomic mass unit (Dalton) is 1/12 of the mass of a carbon atom. Atomic Mass Formula:Īlthough you can specify atomic mass in kilograms, atomic mass is usually expressed in atomic mass units (u), also known as Daltons (Da). Atomic mass can solve stoichiometric problems and calculate the average mass of elements and molecules. Generally, the atomic mass is 1/12 of the mass of carbon 12. It has the masses of three different subatomic particles, such as electrons, protons, and neutrons it is expressed in a unified atomic mass unit, expressed by the unit symbol “U”. What is Atomic Mass?Ītomic mass is defined as the weight/mass of an atom of the chemical element.

In this content, you can learn three different ways of how to find average atomic mass, what is atomicity, Ions, Isotopes with the help of several examples and tables. To determine the atomic mass, it is important to know whether a specific sample is an atom, atomic isotopes in a specific ratio, or a natural sample of elements. An online atomic mass calculator helps you to determine the atomic mass, mass number and atomic symbol.


 0 kommentar(er)
0 kommentar(er)
